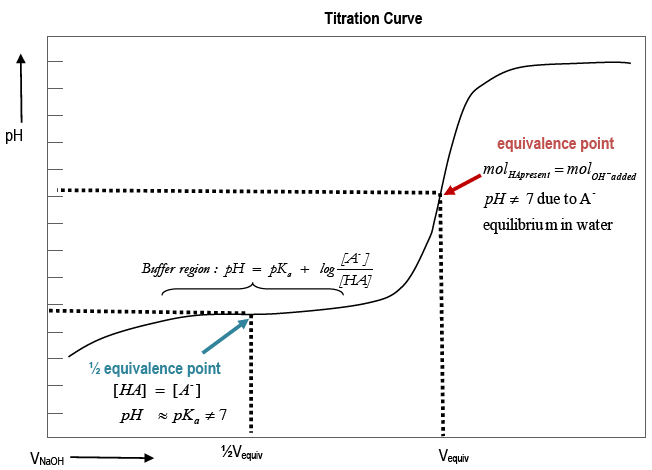
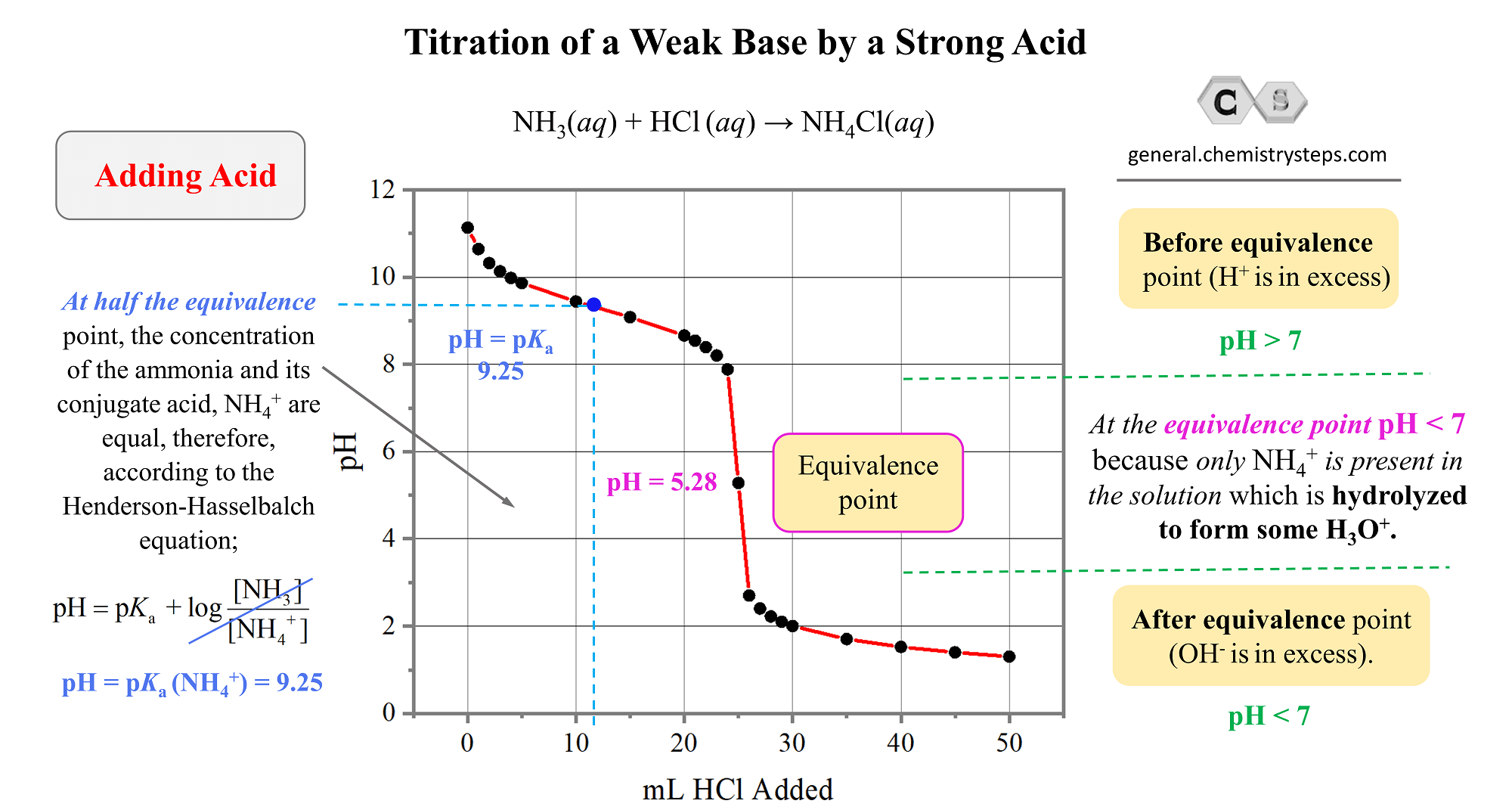
How to Calculate pKa From the Half Equivalence Point in a Weak Acid-Weak Base Titration | Chemistry | Study.com

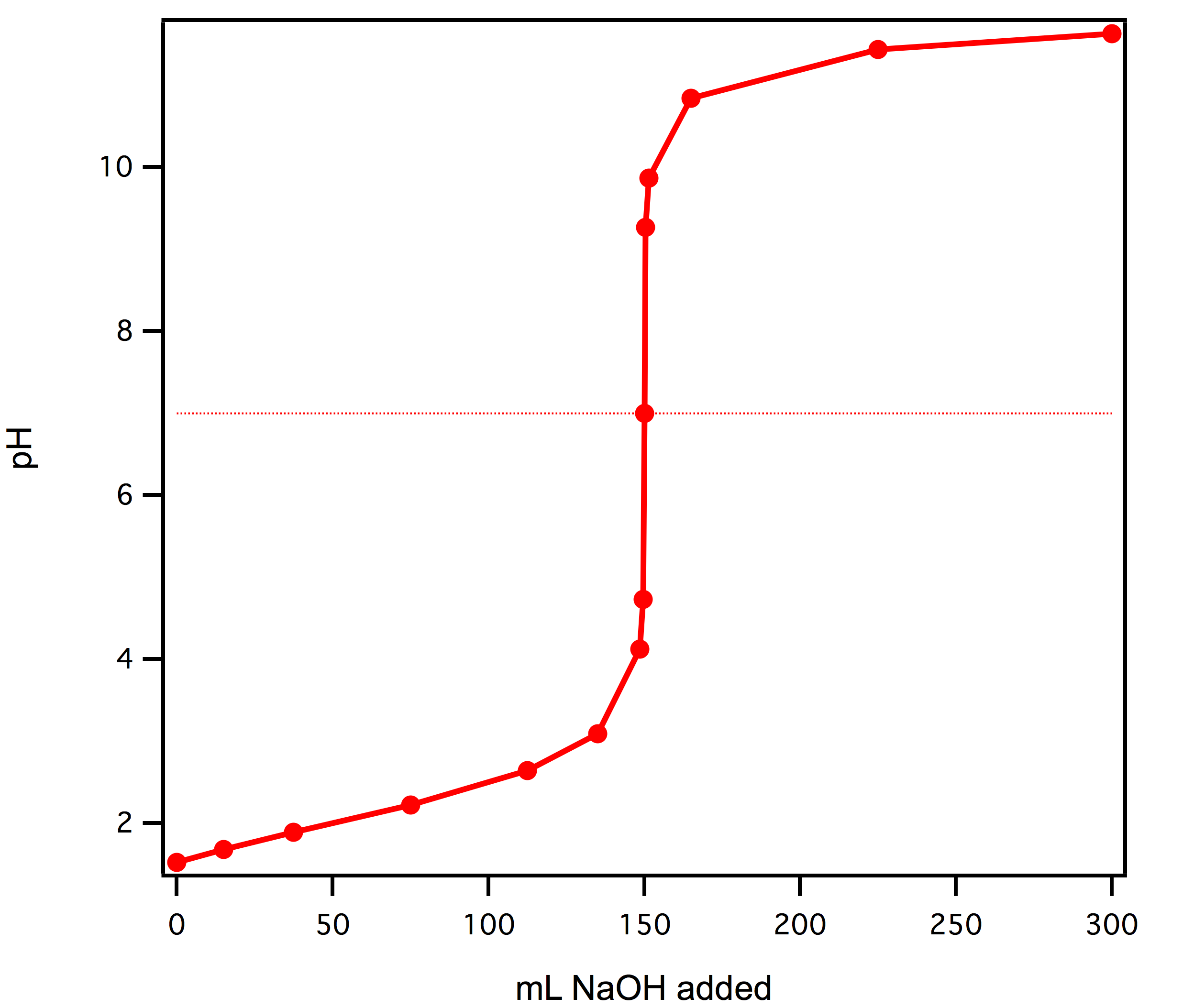
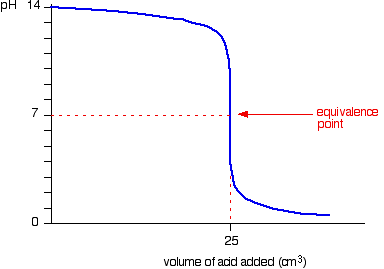
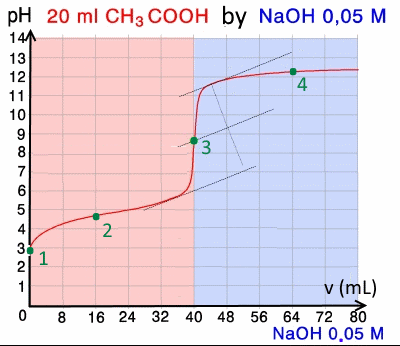
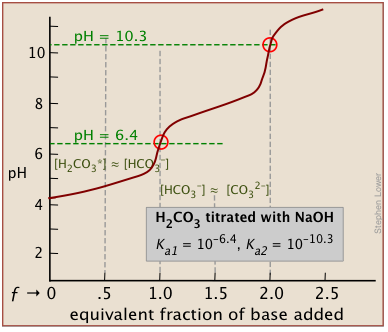
Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how pH varies as 0.100 M NaOH is added to 50.0 mL of 0.100 M HCl.